CRISPR-Associated Transposases (CASTs) make large DNA insertions in Human cells a reality
Tired of only being able to use CRISPR to make small edits? CASTs are here to change that!
Genome editing technologies have improved significantly since the early days of plasmid preps and restriction digests.
That's especially true now with the emergence of programmable nucleases like CRISPR.
These tools enable precise modification of short DNA sequences (<200 bp) and have shown some clinical utility in treating genetic disorders.
But efficiently inserting large DNA sequences (≥1 kb), or even complete genes, at specific genomic sites in human cells is still a challenge.
This limitation is significant because sometimes base editing or CRISPR inactivation of a gene aren’t enough and to really fix a problem we need to replace an entire gene!
CRISPR, unfortunately, can’t do this but can do something similar by triggering double-stranded DNA breaks (DSBs) to promote gene insertion via homology-directed repair (HDR) or alternative methods like homology-independent targeted integration (HITI).
However, HDR is inefficient in non-dividing cells and HITI lacks control over orientation and copy number.
DSBs can also cause harmful genomic byproducts such as indels, large deletions, and chromosomal translocations which makes using CRISPR for large genomic insertions infeasible.
To overcome these issues, researchers have explored CRISPR-associated transposases (CASTs), a bacterial system that allow the insertion of kilobase-scale DNA fragments using Tn7-like transposons.
Type I-F CASTs are particularly attractive due to their high insertion specificity and efficiency in bacteria.
But transferring this activity to human cells has proven difficult because these proteins have very low activity in human cells!
To address this, the researchers used phage-assisted continuous evolution (PACE) to evolve a CAST system that has better performance in human cells.
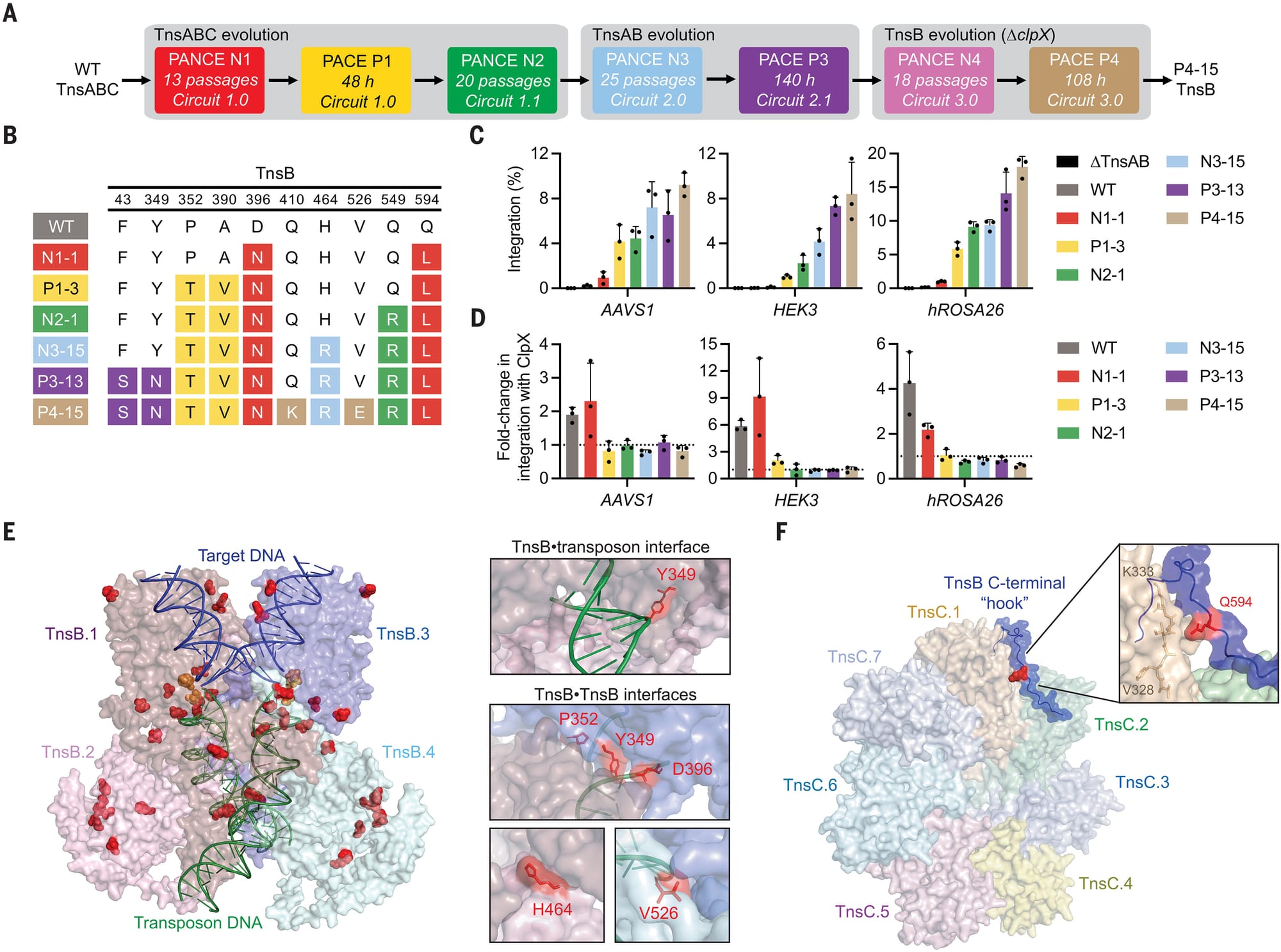
The results of this work can be seen in the figure above:
A) a schematic of the evolution process
B) a table of the mutations found in the best performing transposases
C) shows how well each new tranposase works (higher percent integration is better)
D) the new tranposases don’t require the helper protein ClpX to function efficiently
E and F) space filling models of the engineered proteins highlighting how the mutations interact with DNA.
This evolved system, named evoCAST, achieved up to 30% targeted insertion efficiency at 14 different genomic loci in human cells which is orders of magnitude better than the wild-type system.
CASTs provide an interesting new twist on genome engineering, adding a new tool in our toolkit to help bring more tailored and personalized treatments to the clinic.
