Nerve cells transfer their mitochondria to cancer cells
Just when you thought cancer couldn't get any crazier...neurons can directly transfer their power producing mitochondria to tumor cells
Cancer cells, especially metastatic ones, have exceptionally high energy demands.
To survive in changing and often hostile environments (like those with low oxygen or scarce nutrients) these cells must adapt their metabolism accordingly.
While many tumors rely heavily on glycolysis for energy (inefficient conversion of glucose into 2 ATP), metastatic cells often require the higher energy yield of oxidative phosphorylation (OXPHOS - 32 ATP!) to successfully invade, travel, and colonize distant tissues.
Historically, research on cancer metabolism has focused on how cancer cells internally reprogram their metabolism to survive in these hostile environments.
But it’s increasingly clear that tumors interact closely with their microenvironment to support their metabolic flexibility.
These external interactions with other cells and their environment play a vital role in enabling tumors to adapt and thrive.
For instance, prostate cancer tumor cells have been shown to secrete axon guidance molecules like semaphorin 4F to actively recruit nerves to them!
This process, called neurogenesis, leads to increased innervation and innervated cancers tend to grow faster, become more aggressive, and correlate with worse clinical outcomes.
However, when these cancers are chemically or surgically denervated, tumor growth and metastatic potential decline sharply.
These observations have led researchers to hypothesize that nerves provide more than just signals - they may actually deliver metabolic support directly to cancer cells!
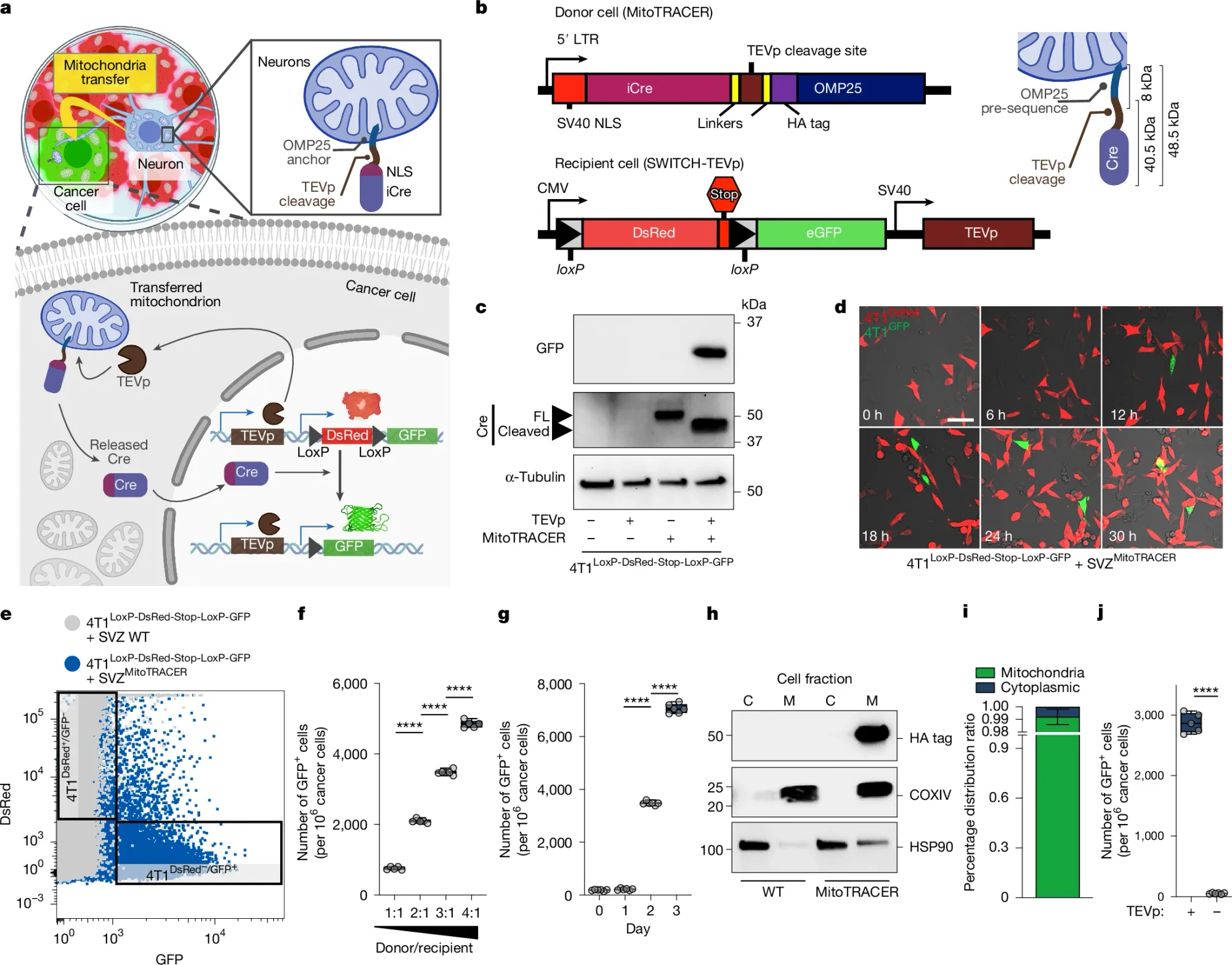
The researchers behind this week's paper built on this idea and developed a genetic tool called MitoTRACER to permanently label cancer cells that receive mitochondria from neurons.
This work can be seen in the figure above a) is a diagram of how the system works - mitochondria in donor neurons are labeled with Cre which is released by TEVp if those mitochondria are transferred into cancer cells that have a dsRed/GFP/TEVp expressing construct. If cancer cells don't get any neuronal mitochondria, they're red, if they do, Cre does its magic and cuts out the dsRed construct allowing the expression of GFP and turning the cells green! b) displays the constructs used in the donor neurons and the cancer cells c) shows that the system works using a western blot d) it works using confocal microscopy e) and with FACS f,g) shows that transfer is both concentration and time dependent and h,i) show that the MitoTRACER components are stuck to the mitochondria and not just floating around the cytoplasm.
Using this system in breast cancer models, it was shown that mitochondria are directly transferred from neurons to cancer cells.
And also that cancer cells that acquired these mitochondria gained enhanced OXPHOS capacity and resistance to metastatic stressors like oxidative damage and mechanical shear.
Further analysis of human tumors confirmed that cancer cells near nerves had greater mitochondrial content, and that denervated tumors had significantly lower mitochondrial load.
These findings solidify the idea that neuronal mitochondria serve as a critical metabolic resource for cancer cells during metastasis.
And if this is true, it means we can use this to our advantage therapeutically!
Because stopping mitochondrial transfer from neurons to tumor cells, inhibiting tumor-induced neurogenesis, or using denervation techniques may significantly impair the ability of tumors to spread and resist therapy.
