Omic.ly Weekly 73
April 28, 2025
Hey There!
Thanks for spending part of your week with Omic.ly!
This Week's Headlines
1) A pig liver makes its way into a human
2) How to do proteomics using a high throughput sequencer
3) There are more than 20 amino acids
Here's what you missed in this week's Premium Edition:
HOT TAKE: Misguided domestic decisions are rocket fuel for China's aspirations to become a biotech powerhouse
Approximately 2,000 people die every year waiting for a liver transplant. Pigs might help change that!
There are approximately 50,000 organ transplants performed every year in the United States.
Of those, about 10,000 are liver transplants.
The liver is a specialized organ that aids in digestion and produces enzymes important for removing toxic substances from our blood stream.
It also produces bile which helps to digest fatty acids and cholesterol.
The liver is the second most transplanted organ behind the kidney.
Organ transplantation is an intense and complicated surgery, but even if the surgery is successful, we still have to deal with the immune system!
We, unfortunately, can't just take an organ from one human and stick it into another because the recipient's body sees that organ as a foreign substance and the immune system treats it like an invader.
This can lead to:
Hyperacute rejection - usually caused by ABO blood typing and HLA mismatches with the body attacking the new organ in minutes to hours.
Acute rejection - this can happen after weeks to months with the recipient's T-cells attacking the donor tissue directly and B-cells making antibodies that tell other immune cells to attack the tissue.
Chronic rejection - constant bombardment from the recipient's immune system leads to a build up of organ damage.
To limit or delay these rejection scenarios, transplant recipients are matched with donors who have similar ABO and HLA profiles.
They're also placed on immunosuppressants to help prevent organ damage after transplantation.
But there aren't enough organs to go around and thousands of people die every year waiting for an organ transplant.
One solution here could be to use animal organs as human organ surrogates.
Pigs have become a popular choice since their organs are the right size and shape as humans' and function similarly.
But we have an even worse rejection problem when it comes to pigs because there's no way to type match a human to a pig!
In recent years, researchers have attempted to get around this problem by humanizing pig organs with genetic engineering to make them "look like" human organs to the immune system of the recipient.
There has been some success with this with other organs such as pig hearts (2 months) and kidneys (6 months).
However, "xenotransplantation" (when you put an organ from one animal into another) had never been done before with a human liver.
That changed a few weeks ago when a brain dead recipient received a genetically modified pig liver.
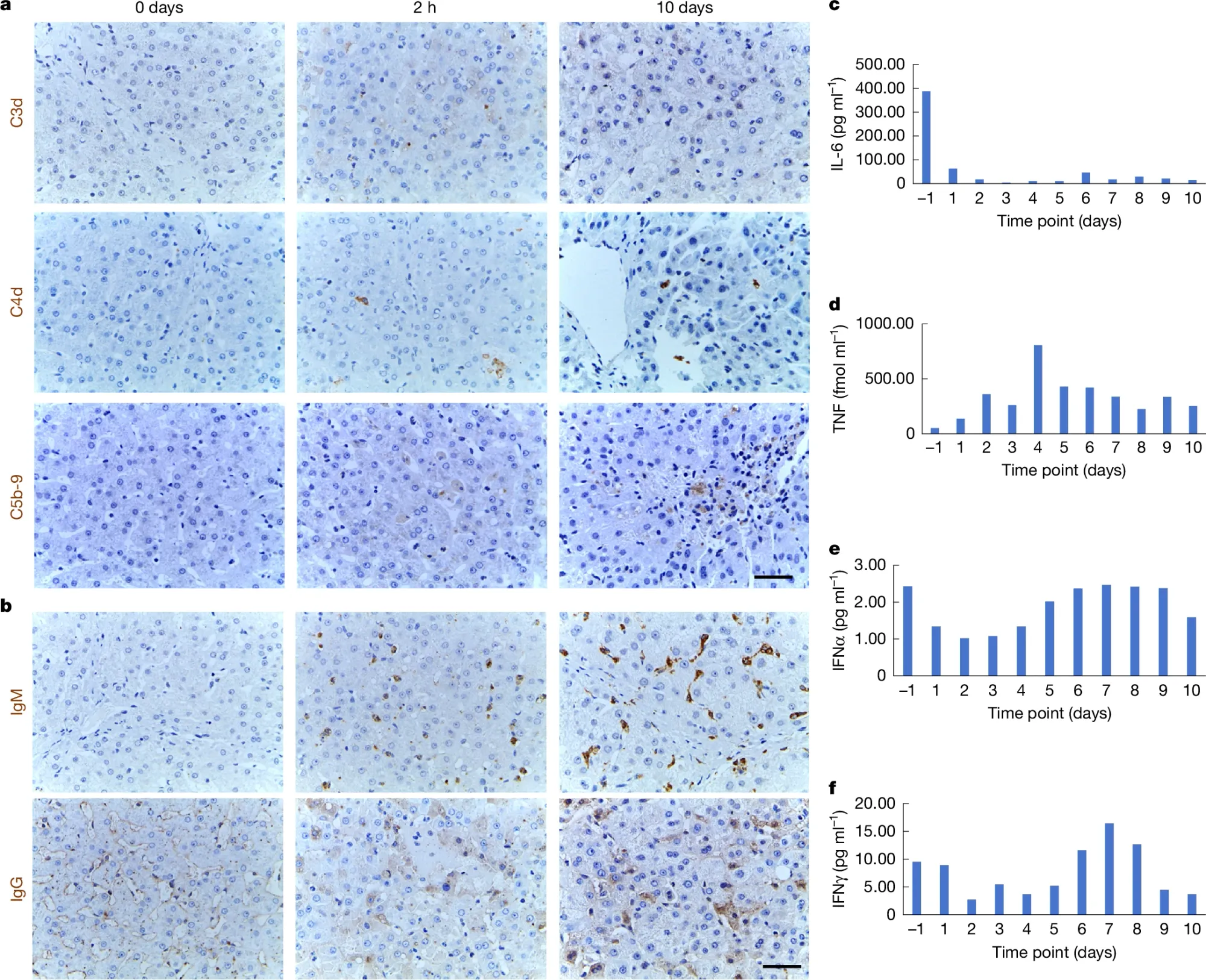
The figure above shows how the patient’s immune system responded to the new liver during the 10 day trial. a) is an immunostain (uses antibodies) of liver tissue and shows little to no deposition (brown bobs) of complement factors (C3d, C4d, C5d-9) which are hallmarks of immune activation; however, b) displays moderate staining for IgG and IgM at 10 days post-transplantation indicating that the humoral immune system may have been activated (they also saw a spike in B cells which were suppressed with rituximab) and in c-f) the major cytokines all appeared to have behaved during the 10 day window suggesting that the immune response was well controlled.
However, detecting IgG and IgM (along with the B cells) was unexpected and was not observed in their previous trials with gene edited pig livers in monkeys.
Despite this immune activation (which would eventually lead to rejection), it could be feasible to use pig livers in humans for short periods of time while they wait for a human transplant to be available.
Overall, this work provides insight into the feasibility, immunological dynamics, and technical challenges of liver xenotransplantation.
It also highlights the need for significantly more research into figuring out how to make pig organs play nice with our immune systems!
###
Tao K-S, et al. 2025. Gene-modified pig-to-human liver xenotransplantation. Nature. DOI: 10.1038/s41586-025-08799-1
Multiplex aptamer/antibody arrays are a gateway drug to get genetics people to embrace proteomics.

When we completed the human genome in 2003, it was heralded as the dawning of a new age in disease prevention.
The truth is that finishing the genome was the first answer in a much longer FAQ about all of the things we didn’t understand in biology.
And since we’ve mostly sorted out the basic code of our genomes, we now need to answer the questions about what all of those genes are doing!
We can do this by looking at other -omes, like the proteome.
But one of the trickiest parts here is that the translation of our genetic code into protein is a massive amplification of that original genetic signal!
There are two copies of each of your genes.
When those are converted into an RNA message, it’s usually not just one RNA that’s made, it can be hundreds.
And then those hundreds of messages can make many thousands of proteins.
So, in genetics we’re usually differentiating the 2 alleles of a gene, but in proteomics we can sometimes be looking for a handful of proteins in a sea of 10,000,000,000!
What this means is that we have to be able to look at way more molecules in proteomics to get the answers we’re looking for than we’ve ever had to look at in genomics.
And it also means the dynamic range is important, or, can the method we’re using detect very abundant and very rare things.
In proteomics we need 10 orders of magnitude, or 1:10,000,000,000 to be the most comprehensive.
We can get pretty close to that using techniques like mass spectrometry, but mass spec instruments are expensive and complicated and most genetics people are infatuated with their sequencers.
So, how can we scam them into doing proteomics while still making them feel like they’re getting good use out of their sequencers?
We can make them use those instruments as counting devices!
Which is a perfect match for two techniques that allow us to detect 10,000+ proteins at a time with the appropriate amount of dynamic range.
Enter Multiplex Aptamer and Immuno Affinity Arrays:
SomaLogic - They do the detecting with aptamers, which are just single stranded pieces of DNA that have been selected for their ability to bind to specific proteins. Detection of said proteins is done by sequencing and counting the aptamers that ended up getting stuck to the proteins in a sample.
Olink - Are more traditional and use antibodies. But these aren't any old antibodies! They've been tagged with DNA! And it's those DNA tags that are then detected and counted on a sequencer.
There are a few other options in this space and all of these techniques are great at counting proteins.
But, their major drawback is that they aren’t able to easily detect protein modifications which are key indicators of a protein's functional state.
Because of this, they’re probably just a bridge, but for now, they’re perfect for getting people excited about the power of proteins!
You might have been told that there are 20 amino acids. That’s a lie.
There are actually about 500 that occur in nature.
But only 22 of them are found in proteins across all organisms.
Of those, 21 appear in humans!
And I know it's killing you to know the name of the 21st amino acid.
But you're going to have to meet Thressa “Terry” Stadtman first.
Stadtman studied two anaerobic bacteria, Clostridium sticklandii and Methanococcus vannielii.
She isolated them from mud that she collected on the shore of San Francisco Bay in 1940.
They served her dutifully throughout her career.
She was fascinated by these microbes because they seemed to be like little chemical magicians!
They were able to perform chemistry at room temperature that would be nearly impossible in a traditional lab.
And they did this chemical magic using enzymes!
One of these was glycine reductase which converts the amino acid glycine to acetyl-phosphate (AcP).
AcP can recharge ADP and turn it into ATP.
If you don’t speak cellular metabolism, this means AcP can be used to literally make energy!
Stadtman was trying to figure out how this worked, but noticed that every so often her bacteria weren’t able to reduce glycine.
She discovered that this occurred whenever one of the components of her enzyme system was missing: protein A.
Thinking this was likely due to a deficiency in the food she used to grow her bacteria, she tested whether the addition of certain nutrients was able to fix the problem.
She found that dumping in Selenium, a trace element, restored protein A production!
But, that posed another question:
Why?
Stadtman decided to answer this by growing her bacteria on radioactive Selenium (75-Se) so she could see where it was going.
She discovered that it actually ended up IN protein A!
She published this finding in 1974, referring to protein A as a ‘selenoprotein.’
But this created another question:
Where was Se hiding?
The figure above provides a pretty definitive answer.
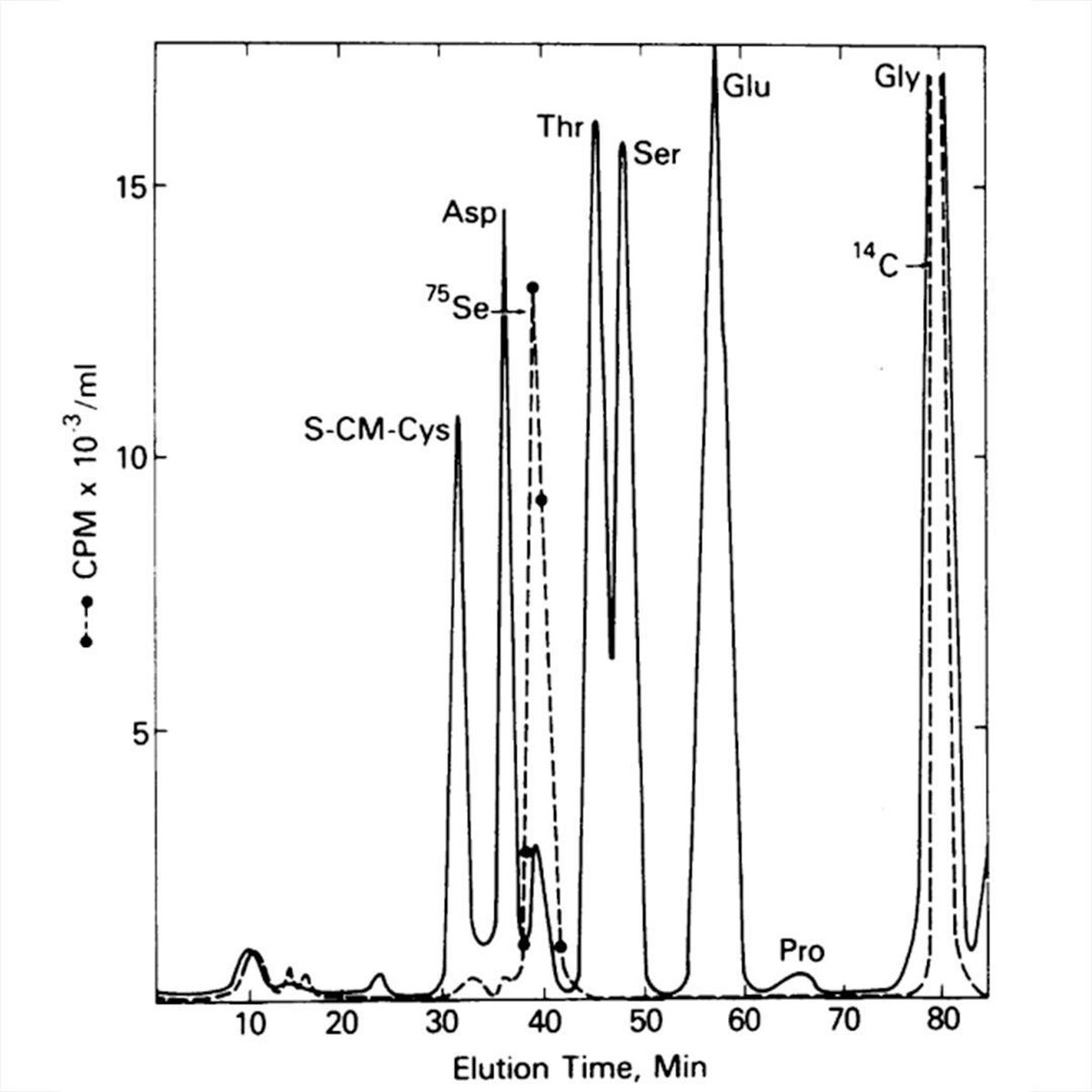
Stadtman grew her bacteria on 75-Se again, isolated protein A, digested it down to its amino acid components, and threw that on a chemical analyzer!
In the plot, the solid line identifies the amino acid components of protein A and the dashed line tracks the radioactive signal.
Stadtman saw that the 75-Se peak coincided with a small unidentified amino acid peak after Aspartate (Asp).
The new peak was too close to Asp to be an Se derivative but she had a hunch that it was a new form of cysteine (S-CM-Cys) which appears first.
This was confirmed in a follow-up experiment!
Not only had Stadtman discovered where the Se went, she also discovered the 21st amino acid:
Selenocysteine!
It was later shown that this rare amino acid is coded for by the UGA stop codon and a special hairpin sequence at the end of a handful of RNAs.
###
Cone JE, et al. 1976. Chemical characterization of the selenoprotein component of clostridial glycine reductase. PNAS. DOI: 10.1073/pnas.73.8.2659
Were you forwarded this newsletter?
LOVE IT.
If you liked what you read, consider signing up for your own subscription here:
