Are personalized vaccines the next big thing in cancer therapy?
The figure kind of speaks for itself.
The therapeutic potential for mRNA technology goes well beyond its use in vaccines for infectious diseases!
Because mRNA is easy to program, it can be used for a variety of other personalized therapies.
One of the most intriguing of these is to use it to eradicate cancers.
This is possible because cancer cells are pretty messed up and they make a lot of weird proteins that can be recognized by the immune system!
These are often referred to as ‘neoantigens.'
That word might look intimidating.
But don't worry, neo is just ‘New’ and antigen is ‘something that can be recognized by the immune system.’
So, how can we use these ‘neoantigens’ to coax the immune system to destroy cancer?
We can make mRNAs that express them, stick them in a vaccine, and prime the immune system to then specifically recognize cancer cells!
But wait, if cancer cells already express these neoantigens, why doesn’t the immune system destroy cancers without the priming?
Mostly it’s because tumors start out small and don’t activate enough cells to mount a strong immune response.
As tumor cells grow, the immune system sees these same antigens over and over again so it begins to tolerate them.
The purpose of a cancer vaccine is to supercharge the immune system to recognize tumors as invaders!
The author’s of today’s paper did just that and targeted a notoriously hard cancer to beat:
Pancreatic ductal adenocarcinoma or PDAC.
This cancer is lethal in 88% of patients and 90% of PDACs recur (come back) 7-9 months after surgery.
So, the need for an alternative therapy is significant.
In this study, patients with a PDAC that could be surgically removed were recruited and then treated with a combination of an immune checkpoint inhibitor, an individualized mRNA neoantigen vaccine, and a chemotherapy regimen.
The mRNA vaccine was designed against tumor-specific neoantigens that were discovered through whole exome and RNA sequencing of the removed PDAC tissue.
Ultimately, 19 patients were administered the checkpoint inhibitor, 16 went on to receive a personalized vaccine, and 15 were given the chemotherapy regimen.
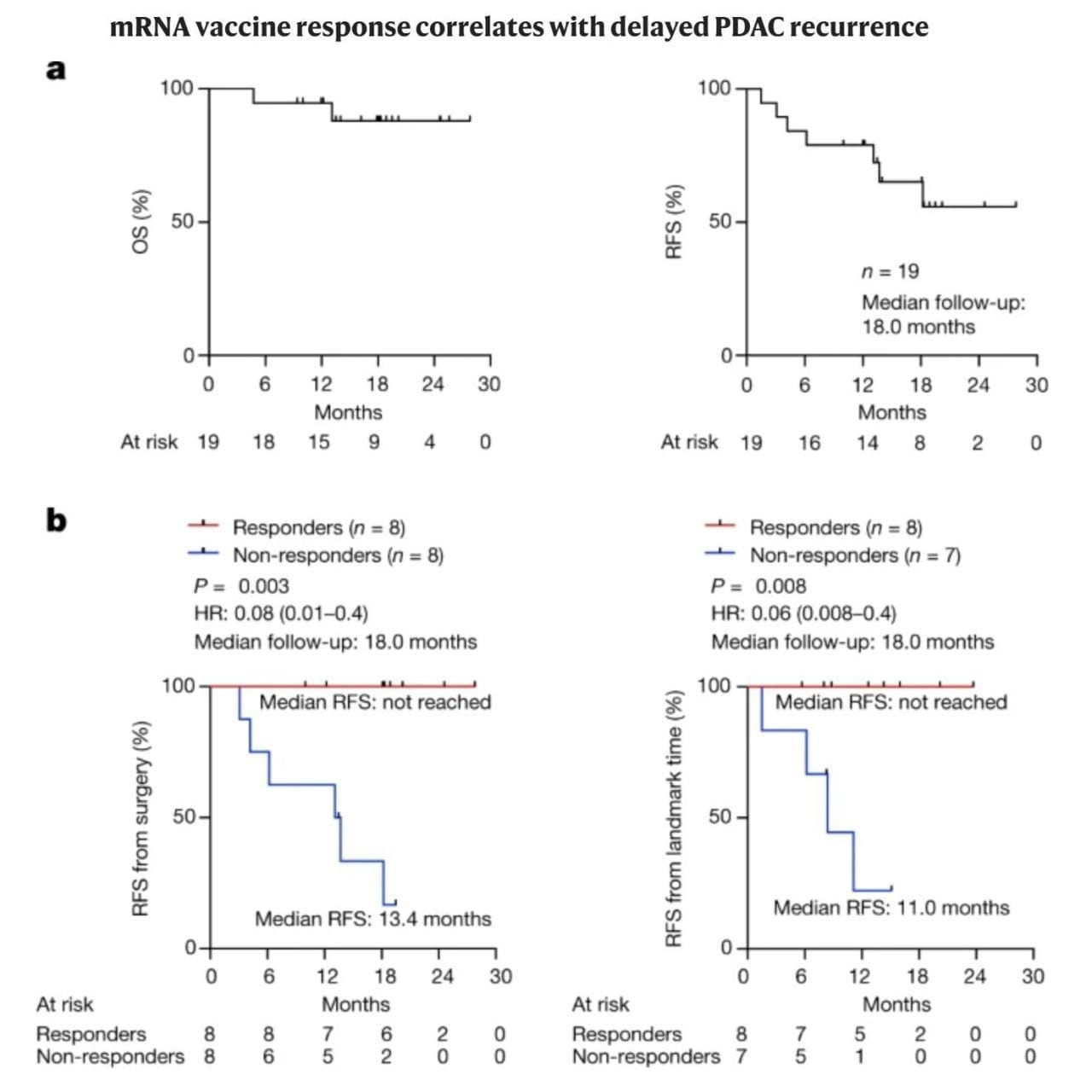
The overall survival and relapse free survival can be seen above in (A), which in itself is a significant improvement over the current standard of care.
But when broken out by vaccine responders and non-responders (B), the results are nothing short of remarkable.
While only 50% of vaccine recipients responded, they were 100% relapse free beyond 18-months.
Despite the small sample size of this initial study, the future of personalized cancer vaccines seems bright.
And, with improved methods for neoantigen identification, hopefully we can get that responder rate closer to 100% too!
